BioLinks Journal Club 3 - Hyperreactivity and Autism Spectrum Disorder
https://www.nature.com/articles/s41593-020-0598-6?draft=marketing
Paper: “Dysfunction of cortical GABAergic neurons leads to sensory hyper-reactivity in a Shank3 mouse model of ASD”
Please read the introduction to this week’s research paper below, which includes the most crucial concepts and background involved. Don’t worry if you don’t understand everything in this summary - we will break it down over the course of the week and dive into the techniques and exact experiments included in the paper . Please remember questions that come up from reading this or anything of particular interest you would like to discuss more on Monday!
Autism Spectrum Disorder refers to a broad range of neurological and developmental conditions that are diagnosed in approximately 1 in 54 children. What differentiates brains from patients with Autism Spectrum Disorder? Research has suggested that certain regions of the brain that are responsible for social action are often activated more in autistic individuals, especially the sensory cortical regions and frontal cortex . One highly observed trait distinction with many cases of autism is sensory over-responsivity to external stimuli, but how these cortical brain regions cause this over-activation and why is largely unknown.
So what causes hyperreactivity to stimuli in autism? The group from this paper investigates this hyperreactivity by using modified mouse models to mimic ASD traits from humans. To do this, the gene for Shank 3B, a protein that the majority of individuals without which have autism, was deleted in the mice for this experiment. This made sense as a reliable model to study as Shank3 deletion in mice had previously yielded autism-like abnormal social interactions and repetitive behaviors. Shank 3B is also vital to excitatory neuron function, which will be important later.
The group first confirmed that this hyperreactivity was indeed observed in their mice lacking Shank3B (knockout) by training normal control mice and Shank 3B knockouts to lick water when their whiskers(vibrissae) were stimulated. They measured factors like how often both types of mice responded correctly to the vibrissae deflection, and how often they licked the water (false positives) even though there was no stimulus. Overall, by combining data for these factors, they confirmed that the Shank 3B knockout mice were responding to the vibrissae stimuli more, and were therefore more reactive than their control counterparts therefore more in terms of sensory response.
This figure explains the possible outcomes of the experiment
above. The green and orange outcomes specifically were observed more in the knockout mice
They next explored what was responsible for these results internally, by looking at the activity of both excitatory and inhibitory neurons in the knockout mice. Excitatory neurons are neurons which release excitatory neurotransmitters into the synapse, which increase the potential difference across the membrane of the neuron (which is negative at rest). This means that excitatory neurotransmitters make neurons more likely to fire and pass on a signal because firing occurs when the membrane potential reaches a high enough threshold level. Conversely, inhibitory neurons release neurotransmitters which lower the potential difference further, making firing less likely and reducing signal. As you can imagine, there is a tight balance between excitatory and inhibitory neurons in neural networks to maintain appropriate amounts of signaling.
This figure highlights the key differences and function of excitatory and inhibitory neurons.
In order to analyze results, what constitutes hyperreactivity inside the body had to be established; it had been previously discovered that the activity of excitatory neurons, specifically the rates at which these neurons fire and the number of times multiple neurons spike at the same time, can be quantifiers of hyperreactivity.
Ultimately, the paper suggests that it was reduction of inhibitory neuron activity in the shank 3B knockout mice that was causing the observed hyperactivity. How? Likely through a process called disinhibition. The findings of this paper serve as an example of this fundamental process, where when the excitatory/inhibitory neuron balance is upset by reduction of inhibition (as observed in the Shank3B knockout mice), in turn resulting in enhanced activity of excitatory neurons (which explains the hyperactivity).
This figure is a demonstration of disinhibition. Pyramidal neurons are excitatory (blue) while GABA interneurons are inhibitory neurons (yellow). When the yellow inhibitory neurons are blocked and their signal is reduced (right), the transmission or activity of the excitatory neuron is increased.
How did they discover these results in the shank 3B knockout mice?
The team first quantified hyperreactivity in the mouse brains by using a technique that allows them to observe when neurons are firing. They suggest that it is higher excitatory neuron activity, including increased firing rates and number of times neurons spike at the same time, in the Shank3B deficient mice that causes the observed hyperreactivity, because their results demonstrated that for both stimulus provoked (whisker deflections) and spontaneous responses, the excitatory neuron activity in the Shank3B deficient mice was higher than the control mice.
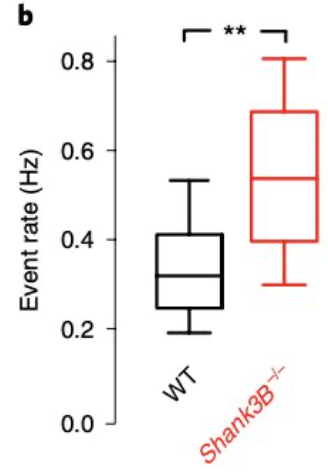
Figure 2b from the paper demonstrates the increased activity in the excitatory neurons (event rate) of the mice lacking shank 3B (red) vs. the control (WT) mice.
But remember that Shank 3B is vital to excitatory neuron function - wouldn’t you expect mice without Shank3B to have impaired excitatory neuron activity? What was causing the increased activity in these neurons in the modified mice? To explore this, the team looked at what was happening to the inhibitory neurons in the Shank3B deficient mice. Using a very similar technique to the previous step, the team looked at factors like neuron firing rates to determine activity in the inhibitory neurons, and found that their activity was actually reduced compared to the control mice.
Figure 3b from the paper demonstrates decreased activity (event rate) in the inhibitory neurons of the mice lacking shank 3B (red) vs. the control (WT) mice.
Was it actually inhibitory neuron activity reduction at the root of the hyperreactivity observed in the Shank3B negative mice? The group found out that the hyperexcitability of the excitatory neurons
discovered before was likely due to the inhibitory reduction. They determined this by using a technique that selectively deleted the Shank3B from the inhibitory neurons alone, and then looked at the effect of the deletion on the excitatory neurons in the same mice (which still contained Shank3B). These results were very similar to the result when Shank3B was deleted from all the neurons and hyperactivity of the excitatory neurons was seen, suggesting that the inhibitory reduction was what led to excitatory hyperactivity. (See disinhibition model above)
This paper suggests that the reduction of inhibitory neuron activity is at the root of the hyperreactivity in the shank3B knockout mice. If disinhibition is indeed what is causing hyperreactivity in ASD, this study has huge implications for potential therapies. The paper further validates Shank3 knockouts as both a model for ASD in future experiments and as a significant contributor to a common symptom of autism.